Have you ever wondered what powers the sun or how scientists aim to create nearly limitless clean energy here on Earth? The answer lies in two special forms of hydrogen: deuterium and tritium.
Understanding these tiny atoms could change the way you see energy and the future of our planet. You’ll discover what makes deuterium and tritium so unique, why they matter, and how they might unlock incredible possibilities for you and everyone else.
Ready to dive into the fascinating world of these powerful elements? Let’s get started.
Deuterium Basics
Deuterium is a special form of hydrogen. It has unique properties that make it important in science and energy. Understanding deuterium basics helps us see its role in fusion and other fields.
Let’s explore what makes deuterium different, where it is found, and how we get it.
Atomic Structure
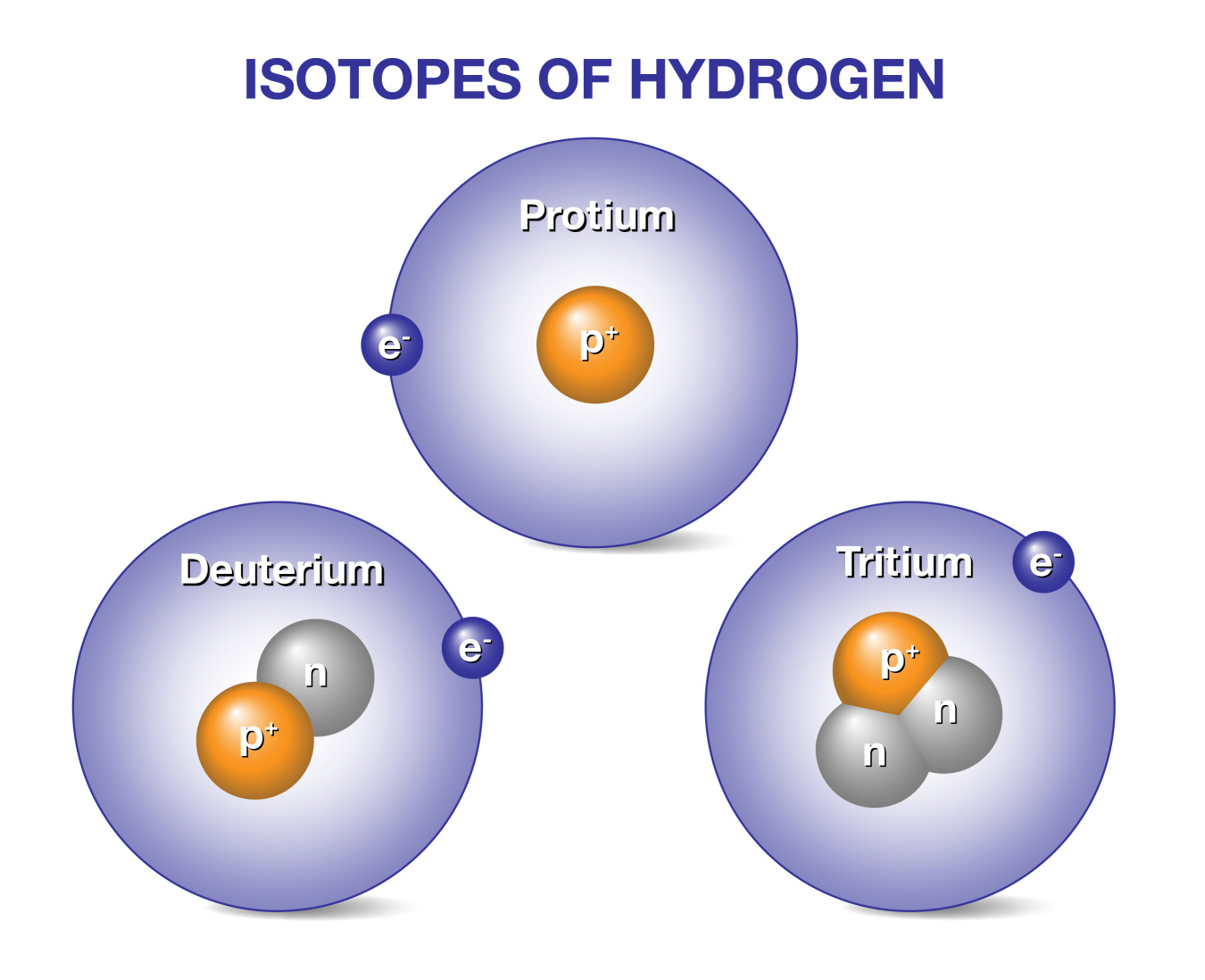
Deuterium has one proton and one neutron in its nucleus. Regular hydrogen has only one proton. This extra neutron makes deuterium heavier. Its atomic symbol is D or ²H. This difference changes its behavior in chemical reactions.
Natural Occurrence
Deuterium occurs naturally in water and the atmosphere. It makes up about 0.015% of all hydrogen on Earth. Most deuterium is found in oceans. It exists as heavy water when combined with oxygen. This natural presence helps scientists study nuclear fusion.
Extraction Methods
Extracting deuterium requires separating it from regular hydrogen. One method uses electrolysis to split water into hydrogen and oxygen. Heavy water is collected because it contains more deuterium. Another way is distillation, which separates heavy water by boiling points. These methods provide the deuterium needed for research and industry.
Tritium Insights
Tritium is a special form of hydrogen. It has unique features that make it important in science and energy. This section explains the key facts about tritium. You will learn about its radioactive nature, how it is made, and the problems with storing it safely.
Radioactive Properties
Tritium is a radioactive isotope of hydrogen. It emits low-energy beta particles. These particles cannot pass through skin but can harm if ingested. Tritium has a half-life of about 12.3 years. This means it slowly loses radioactivity over time. Its radiation is weak but needs careful handling.
Production Techniques
Tritium is not common in nature. It is mostly made in laboratories or nuclear reactors. One method uses neutron bombardment on lithium. Another way is through heavy water reactors. Producing tritium requires precise control and special equipment. These methods ensure a steady supply for research and energy uses.
Storage Challenges
Storing tritium safely is difficult. It is a gas that can escape easily. Tritium can leak through many materials. Containers must be airtight and strong. Special tanks made from metal or glass are common. Careful monitoring is needed to prevent leaks. Safe storage protects people and the environment.
Fusion Energy Potential
Fusion energy offers a clean and nearly limitless source of power. It mimics the process that fuels the sun. Scientists focus on deuterium and tritium to make fusion possible on Earth. These elements hold the key to unlocking vast amounts of energy with minimal waste. Understanding how fusion works and the role of these fuels is essential to grasping its potential.
How Fusion Works
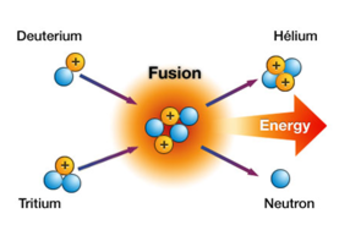
Fusion joins two light atomic nuclei into one heavier nucleus. This process releases a huge amount of energy. It requires extremely high temperatures and pressure to overcome repulsion. The nuclei must collide with enough force to fuse. This reaction powers stars and can generate electricity on Earth.
Role Of Deuterium And Tritium
Deuterium and tritium are heavy forms of hydrogen. Deuterium has one proton and one neutron. Tritium has one proton and two neutrons. These isotopes fuse more easily than others, needing less energy to start. Deuterium is abundant in seawater, making it widely available. Tritium is rare but can be bred inside fusion reactors. Their combination produces the highest energy output in fusion reactions.
Energy Output And Efficiency
Fusion of deuterium and tritium releases energy millions of times greater than burning fossil fuels. The process produces no greenhouse gases or long-lived radioactive waste. Energy from fusion is clean and sustainable. Fusion reactors aim to produce more energy than they consume. Achieving this balance is crucial for future practical fusion power plants.
Current Fusion Technologies
Current fusion technologies focus on using deuterium and tritium as fuel. These two hydrogen isotopes fuse to release huge amounts of energy. Scientists use different methods to create the extreme conditions needed for fusion. The goal is to produce clean, almost limitless energy.
Tokamak Reactors
Tokamak reactors use strong magnetic fields to hold hot plasma. The plasma contains deuterium and tritium nuclei. These nuclei collide and fuse at very high temperatures. Tokamaks have a doughnut-shaped chamber to keep the plasma stable. The International Thermonuclear Experimental Reactor (ITER) is a famous tokamak project. It aims to show fusion energy can work on a large scale.
Inertial Confinement
Inertial confinement fusion uses lasers or particle beams. These beams hit a small fuel pellet with deuterium and tritium. The pellet compresses and heats rapidly. This creates conditions for fusion before the pellet blows apart. This method tries to mimic the fusion process inside stars. It is fast and happens in a tiny space.
Alternative Approaches
Other fusion methods explore different ways to achieve fusion. Some use magnetic fields but with different shapes than tokamaks. Others use electric fields to heat and compress the fuel. These approaches try to solve challenges like plasma instability or cost. They are less developed but could offer new paths to fusion energy.
Fuel Supply And Sustainability
The fuel supply and sustainability of deuterium and tritium are key to fusion energy’s future. Understanding how these fuels are sourced and managed helps us see the potential of fusion as a long-term energy solution. This section breaks down the availability, production, and environmental effects of these fuels.
Availability Of Deuterium
Deuterium is a stable form of hydrogen found naturally in water. It makes up about 0.015% of all hydrogen atoms in oceans. This means the supply is vast and almost unlimited for human use. Extracting deuterium from water is simple and does not harm the environment. This abundant availability makes deuterium a reliable fuel source for fusion reactors.
Tritium Breeding
Tritium is rare and not found in large amounts on Earth. Fusion reactors produce tritium by using lithium in a process called breeding. Neutrons from fusion reactions hit lithium atoms, creating tritium. This method can provide a steady supply inside the reactor. Tritium breeding is essential because it reduces reliance on external sources and supports continuous fusion energy production.
Environmental Impact
Deuterium extraction has a low environmental footprint since it comes from seawater. Tritium breeding involves lithium, which is more limited but recyclable inside reactors. Fusion fuel does not produce greenhouse gases or long-lived radioactive waste. This makes fusion fuel supply cleaner and safer than fossil fuels. The sustainable cycle of deuterium and tritium supports a greener energy future.
Challenges And Solutions
Deuterium and tritium play a key role in nuclear fusion research. Using these isotopes faces many challenges. Scientists work hard to find strong solutions. These focus on handling extreme conditions safely and efficiently.
Containment Issues
Containing deuterium and tritium at high temperatures is tough. Fusion reactions need heat hotter than the sun. No solid container can hold such heat. Magnetic fields trap the plasma and keep it stable. Researchers improve these fields to avoid leaks and losses. Better containment means safer and longer fusion reactions.
Material Durability
Materials face constant damage from neutron radiation. These neutrons come from fusion reactions. They weaken metals and cause cracks. Scientists test new alloys and ceramics to resist damage. Durable materials help build longer-lasting fusion reactors. This reduces maintenance and increases efficiency.
Safety Concerns
Tritium is radioactive and requires careful handling. It can leak into the environment if not controlled. Strict safety rules protect workers and surroundings. Monitoring systems detect leaks early and prevent harm. Safe fusion power depends on managing these risks well.
Future Prospects
The future of deuterium and tritium in energy production holds great promise. These hydrogen isotopes are key to fusion energy, a potential source of clean power. Scientists and engineers work hard to make fusion practical and affordable. Progress in research and technology drives hope for new energy solutions.
Understanding the future prospects of deuterium and tritium involves looking at important milestones, expected timelines for use, and their effect on global energy markets.
Research Milestones
Scientists have made steady progress in fusion research. Experiments show that deuterium and tritium can create energy through fusion. New machines test ways to control the fusion process safely. Each step brings us closer to stable energy output from fusion reactors.
Commercialization Timeline
Commercial fusion energy using deuterium and tritium may emerge in the next 20 to 30 years. Building fusion power plants requires solving many technical challenges. Pilot plants are expected to prove fusion’s viability for electricity. Gradual adoption will follow as costs decrease and systems improve.
Global Energy Impact
Fusion energy could reduce dependence on fossil fuels significantly. Deuterium is abundant in seawater, ensuring a vast fuel supply. Tritium is rarer but can be bred in reactors, supporting sustainability. Fusion’s clean nature offers a future with less pollution and more energy security worldwide.
Frequently Asked Questions
What Are Deuterium And Tritium In Simple Terms?
Deuterium and tritium are isotopes of hydrogen. Deuterium has one neutron, while tritium has two. Both are used in nuclear fusion research for clean energy.
Why Are Deuterium And Tritium Important For Fusion?
They serve as fuel in fusion reactions. When combined, they release massive energy, mimicking the sun’s power. This makes them crucial for future energy solutions.
How Is Deuterium Extracted From Water?
Deuterium is separated through electrolysis or distillation of water. It naturally occurs in small amounts in seawater, making it a sustainable resource for fusion fuel.
What Safety Concerns Exist With Tritium?
Tritium is radioactive but emits low-energy beta radiation. Handling requires caution to avoid ingestion or inhalation. It poses minimal external radiation risk.
Conclusion
Deuterium and tritium play key roles in nuclear science. They are forms of hydrogen with different neutron counts. Deuterium is stable, while tritium is radioactive. Both are used as fuel in fusion reactions. Fusion promises cleaner energy for the future.
Understanding these isotopes helps us learn about energy and matter. Their unique properties make them important in research. Scientists continue to explore their potential every day. The study of deuterium and tritium is both fascinating and useful.
Apply for this vacancy
For more information
For more information, please don’t hesitate to contact us. We’re here to assist with any questions or provide additional details to help you make informed decisions. Reach out today, and let’s connect!
Please mention the respective article number.
For more information
For more information, please don’t hesitate to contact us. We’re here to assist with any questions or provide additional details to help you make informed decisions. Reach out today, and let’s connect!
Please mention the respective article number.

